
| Report Code: PP10153 | Published: January 2019 | Pages: 74 | Available format: |
| Therapeutic Area(s): | Others | Report Type: Indication Pipeline Reports |
Overview
Achondroplasia, also termed as ACH and achondroplastic dwarfism, is an autosomal dominant genetic disorder that leads to short-stature/ limb dwarfism. It is caused due to abnormal cartilage formation. Cartilage is responsible for the development of skeleton and initial bone growth. In this disease, the ossification process (conversion of cartilage to bone) stops in long bones, such as arms and legs, which causes achondroplasia.
ACHONDROPLASIA THERAPEUTICS UNDER DEVELOPMENT (2018)
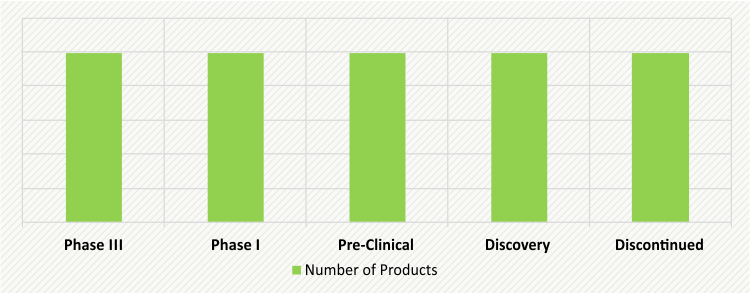
Majority of drug candidates in the pipeline are being developed with the use of advanced technologies, which enable targeted delivery of drugs or protect drugs from degrading in the body before reaching the target. By leveraging these technologies, the companies are developing biological and synthetic drugs to prevent the progression of achondroplasia.
Furthermore, positive clinical trial results and patent approvals have been facilitating the drug development process. These positive clinical trials and patent approvals have been further helping the players in the achondroplasia pipeline to achieve different milestones, such as grant and designations, from the government regulatory bodies and institutes including, U.S. Food and Drug Administration (USFDA), European Medicines Agency (EMA), and National Institute of Health (NIH) among others.
Pipeline Analysis
Competitive Landscape
Some of the key players involved in the development of achondroplasia therapeutics include BioMarin Pharmaceutical Inc., Ascendis Pharma A/S, and Therachon AG.