| Report Code: PP10232 | Report Type: Indication Pipeline Reports | Pages: 300+ | Available format: |
| Therapeutic Area(s): | Gastroenterology |
Overview
Constipation refers to an irregular bowel movement, which is characterized by lumpy and hard stool, pain in passing the stool, and feeling of incomplete emptying. Additional implications of constipation include hemorrhoids anal fissure, rectal prolapse, and fecal imprecation.
Generally, constipation results from the use of medications such as calcium containing antacids, iron supplements, diuretics, and others. Individuals who have low intake of water and fibers are more prone to constipation. Physical examination, endoscopy, and colorectal transit studies are some of the most commonly diagnostic technique used to diagnose constipation.
Positive clinical trial results and strategic decisions of companies to collaborate with other companies are also facilitating drug development in constipation therapeutics pipeline arena. Additionally, the issuance of patents helps in achieving different milestones in the form of grants and designations from regulatory bodies and institutes, including the U.S. Food and Drug Administration (USFDA), the European Medicines Agency (EMA), and the National Institutes of Health (NIH).
Pipeline Analysis
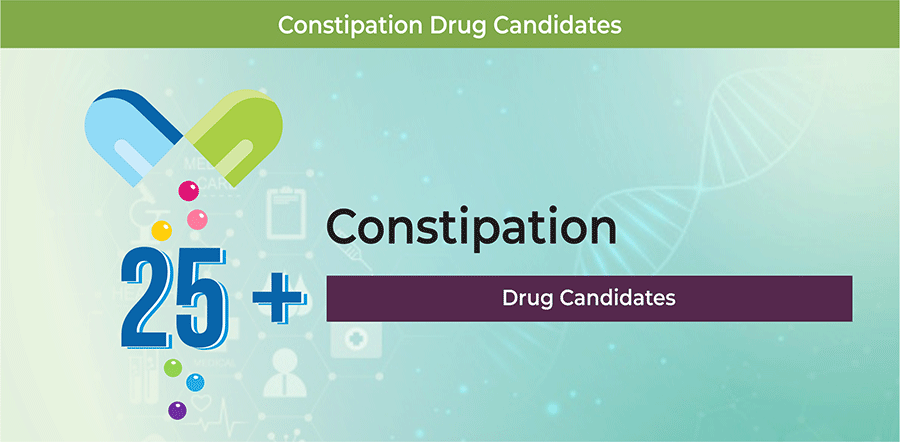
As of February 2019, the constipation therapeutics pipeline comprises 25+ therapeutic candidates in different stages of development.
Epidemiology Analysis
The report provides epidemiology forecast of constipation for seven major markets, such as the U.S., Japan, and EU5 countries (the U.K., Germany, France, Italy, and Spain). It covers prevalent and treated patient population for the period 2016–2028. According to an article published in the journal Medicine in 2018, the estimated average prevalence of constipation was 16% worldwide; whereas in people aged 60–110 years, it was 33.5%.
Competitive Landscape
Some of the key players involved in the development of constipation therapeutics in the late and mid-stage, include Eisai Co. Ltd., Synergy Pharmaceuticals Inc., Kyowa Kirin Pharmaceutical Development Ltd., Ironwood Pharmaceuticals Inc., Forest Laboratories Inc., AstraZeneca PLC, and Sucampo Pharmaceuticals Inc.
Report Insights
Some highlights of the report, “Constipation Therapeutics – Competitive Landscape, Epidemiology Forecast, and Pipeline Analysis, 2019”, include: